Your cart is currently empty!
Resource Details
Journal of Proteome Research
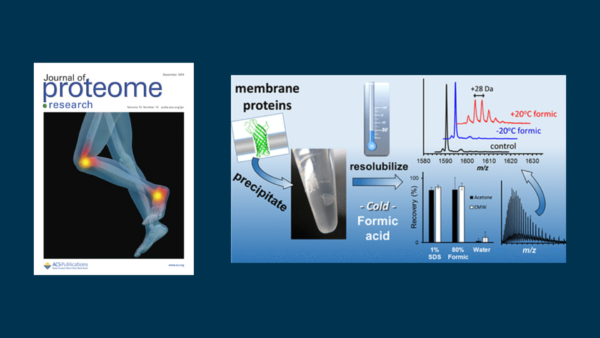
Resolubilization of Precipitated Intact Membrane Proteins with Cold Formic Acid for Analysis by Mass Spectrometry
by Alan A. Doucette, Douglas B. Viera, Dennis J. Orton, & Mark J. Wall
Protein precipitation in organic solvent is an effective strategy to deplete sodium dodecyl sulfate (SDS) ahead of MS analysis. Here we evaluate the recovery of membrane and water-soluble proteins through precipitation with chloroform/methanol/water or with acetone (80%). With each solvent system, membrane protein recovery was greater than 90%, which was generally higher than that of cytosolic proteins. With few exceptions, residual supernatant proteins detected by MS were also detected in the precipitation pellet, having higher MS signal intensity in the pellet fraction. Following precipitation, we present a novel strategy for the quantitative resolubilization of proteins in an MS-compatible solvent system. The pellet is incubated at −20 °C in 80% formic acid/water and then diluted 10-fold with water. Membrane protein recovery matches that of sonication of the pellet in 1% SDS. The resolubilized proteins are stable at room temperature, with no observed formylation as is typical of proteins suspended in formic acid at room temperature. The protocol is applied to the molecular weight determination of membrane proteins from a GELFrEE-fractionated sample of Escherichia coli proteins.