Your cart is currently empty!
Resource Details
Journal of Proteome Research
Automated SDS Depletion for Mass Spectrometry of Intact Membrane Proteins Though Transmembrane Electrophoresis
by Carolyn Kachuk, Melissa Faulkner, Fang Liu & Alan A. Doucette
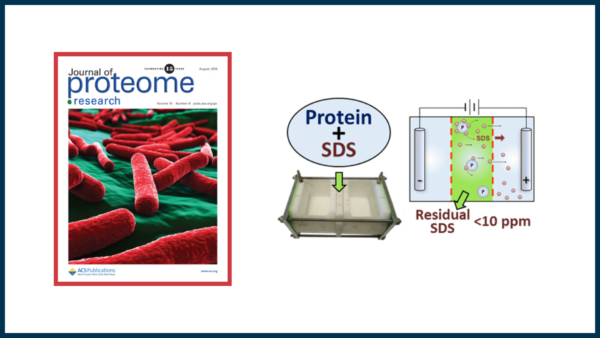
Membrane proteins are underrepresented in proteome analysis platforms because of their hydrophobic character, contributing to decreased solubility. Sodium dodecyl sulfate is a favored denaturant in proteomic workflows, facilitating cell lysis and protein dissolution; however, SDS impedes MS detection and therefore must be removed prior to analysis. Although strategies exist for SDS removal, they provide low recovery, purity, or reproducibility. Here we present a simple automated device, termed transmembrane electrophoresis (TME), incorporating the principles of membrane filtration, but with an applied electric current to ensure near-complete (99.9%) removal of the surfactant, including protein-bound SDS. Intact proteins are recovered in solution phase in high yield (90-100%) within 1 h of operation. The strategy is applied to protein standards and proteome mixtures, including an enriched membrane fraction from E. coli, resulting in quality MS spectra free of SDS adducts. The TME platform is applicable to both bottom-up MS/MS as well as LC-ESI-MS analysis of intact proteins. SDS-depleted fractions reveal a similar number of protein identifications (285) compared wit a non-SDS control (280), being highly correlated in terms of protein spectral counts. This fully automated approach to SDS removal presents a viable tool for proteome sample processing ahead of MS analysis. Data are available via ProteomeXchange, identifier PXD003941.