Your cart is currently empty!
Resource Details
Journal of Chromatography A
Comparison of Sodium Dodecyl Sulfate Depletion Techniques for Proteome Analysis by Mass Spectrometry
by Carolyn Kachuk, Kegan Stephen & Alan A. Doucette
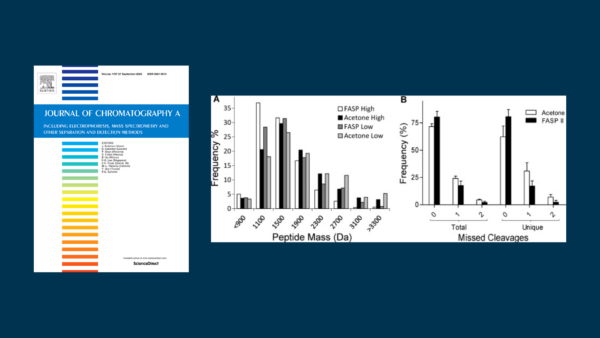
In proteomics, sodium dodecyl sulfate (SDS) is favored for protein solubilization and mass-based separation (e.g. GELFrEE or SDS PAGE). Numerous SDS depletion techniques are available to purify proteins ahead of mass spectrometry. The effectiveness of the purification has a controlling influence on the success of the analysis. Here we quantitatively assess eight approaches to SDS depletion: in-gel digestion; protein precipitation in acetone or with TCA; detergent precipitation with KCl; strong cation exchange; protein level and peptide level purification with Pierce detergent removal cartridges; and FASP II. Considering protein purity, FASP II showed the highest degree of SDS removal, matching that of in-gel digestion (over 99.99% depleted). Other methods (acetone, strong cation exchange, Pierce cartridges) also deplete SDS to levels amenable to LC-MS (>99%). Accounting for protein recovery, FASP II revealed significant sample loss (<40% yield); other approaches show even greater protein loss. We further assessed acetone precipitation, having the highest protein recovery relative to FASP II, to process GELFrEE fractionated Escherichia coli ahead of bottom-up mass spectrometry. Acetone precipitation yielded a 17% average increase in identified proteins, and 40% increase in peptides, indicating this approach as a favored strategy for SDS depletion in a proteomics workflow.